Hymon SARS-CoV-2 Test Kit.pptx
Hymon SARS-CoV-2 Test Kit.pptx
Hymon SARS-CoV-2 Test Kit is produced by HymonBio Co., LTD, which is founded by PhDs from University of Texas & Chinese Academy of Sciences and focus on precision testing on cancer gene screening, epidemic pathogen DNA/RNA tests. Polyhex Technology Co., Ltd. is authorized to marketing development, sales and product support of Hymon SARS-CoV-2 Test Kit overseas from March 13th, 2020 to March 13th, 2021.
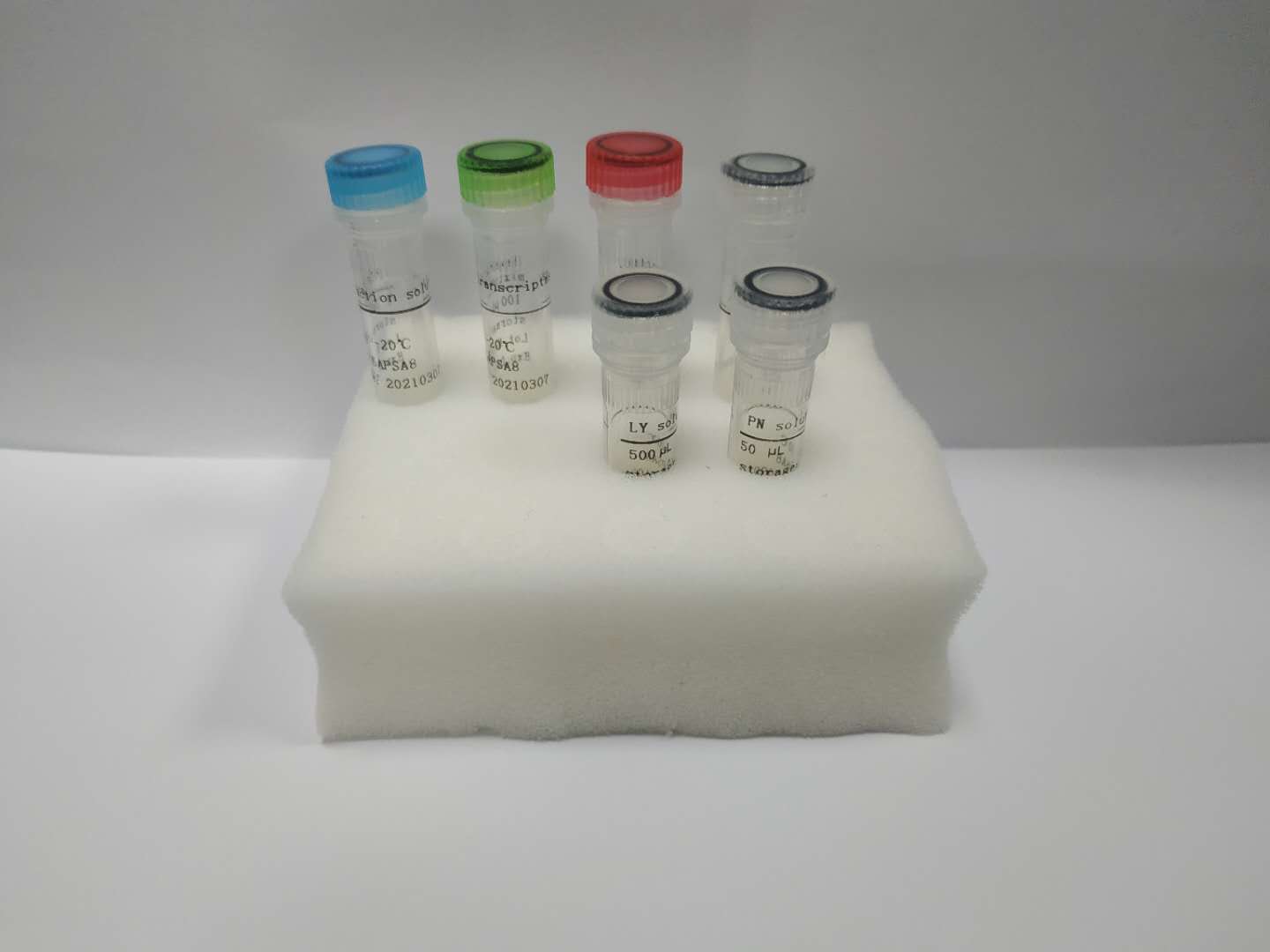
Hymon SARS-CoV-2 Test Kit provides in vitro detection of coronavirus SARS-CoV-2 in nasopharyngeal swab and other respiratory samples. The virus infects human and caused COVID-19.
The test applies proprietary MoLock, a nanobody-based probe technology, and OT, a buffer system technology, to build the multiplexing RT-PCR kit that targets N and E genes of SARS-CoV-2 to achieve ultrasensitive and robust detection of viral RNA in a same-tube, sample-to-result process. With overall turnaround time at 1.5 hours for 96 tests, the kit offers 5 copies/PCR detection limit of SARS-CoV-2 with high specificity and maintains a consistent quality with an internal control. Hymon SARS-CoV-2 Test Kit provides references to key clinical decisions such as confirmation of early infection, evaluation of therapeutic efficacy, and determination of negative conversion.
Features:
High accuracy
High cost performance
Quickly detect the Coronavirus infection
Quickly evaluate the Coronavirus treatment effect
Already applied in Wuhan, Nanjing, Xi'an and other major cities in China.
Clinical sample quality control test report available (Shanghai Clinical Laboratory Center)
Fast delivery (Certificates for exportation and sale transport of chemical goods are available)